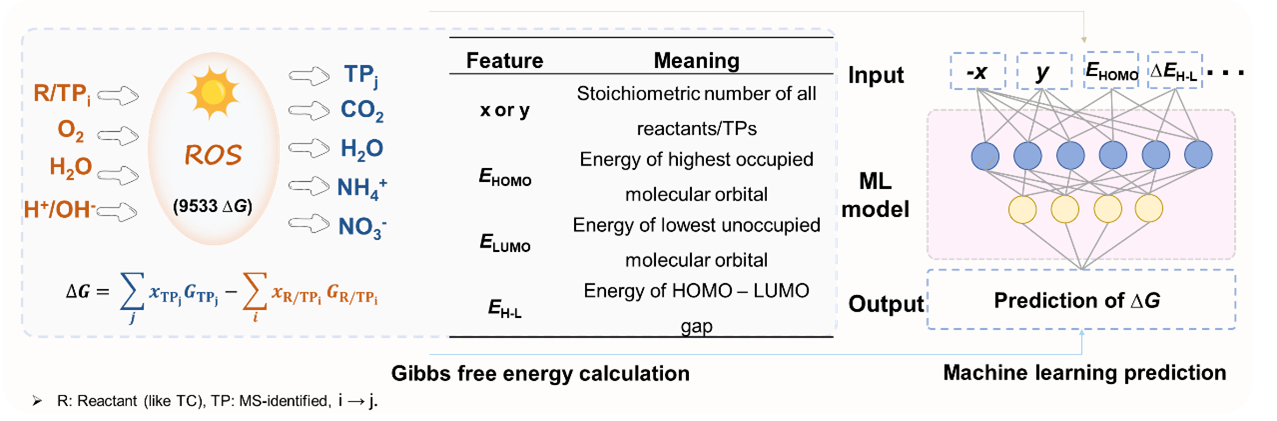
Gibbs Free Energy Changes (ΔG) of Tetracycline Degradation Dataset
A mechanistic reaction database was constructed, encompassing 120 overall stoichiometrically balanced reactions and 9,533 elementary reaction steps relevant to the photocatalytic degradation of tetracycline and related compounds. For each reaction step, both thermodynamic data and reaction descriptors were compiled to enable machine learning analysis of transformation energetics. Each reaction entry includes:
• A set of stoichiometric descriptors, where x and y represent the stoichiometric coefficients of reactants and products, respectively. Species are categorized as follows: target transformation products (TPi, TPj), environmental species (O2, H2O, CO2, NH4+, NO3-), ROS (1O2, O2•-, •OH), and photogenerated charge carriers (e-, h+). Note: Stoichiometric coefficients are signed (x < 0 for reactants, y > 0 for products) and may include fractional values (e.g., 0.5 O₂) to preserve thermodynamic consistency.
• A set of quantum chemical features, including: energy of highest occupied molecular orbital (EHOMO,TPi, EHOMO,TPj), energy of lowest unoccupied molecular orbital (ELUMO,TPi, ELUMO,TPj), and energy of HOMO-LUMO gap (∆EH-L,TPi, ∆EH-L,TPj,).
• The Gibbs free energy change (ΔG), computed using DFT (M06-2X/6-311+G(d,p) or def2-SVP for polymers; SMD solvation model, 298.15 K, 1 atm).
To request the datasets, please register and complete the
LICENSE FORM,
scan and email it to majing@nju.edu.cn.